Low Temperature Conductivity and Structural Analysis of Cu-CeO₂ Nanoparticles
- Quantitative data withheld pending publication; qualitative trends summarized
I was fortunate to work with Professor Siu-Wai Chan and Xinyi Ma on this project during my senior year and continuing into my master’s studies. I am also grateful for the guidance and support of Professor Simon Billinge and Dr. Adrian Chitu. The pellet pressing was carried out primarily at the Lamont–Doherty Earth Observatory, and I thank Professor David Walker and Supervisor Echo Shuo for their generous assistance.
The growing demand for functional nanomaterials in energy conversion and sensing has motivated extensive efforts to tailor their electrical behavior through controlled defect and dopant engineering. Previous studies from our group on copper-doped nanoceria demonstrated that samples with approximately 8 % Cu exhibited the most favorable catalytic performance at 150-250°C, although the underlying mechanism remained unclear. The present work seeks to provide insight into the charge-transport processes that may contribute to this behavior.
Sample Preparation
The nanoparticle samples were synthesized via coprecipitation at room temperature (around 25.5 °C).
Reactants
- Cerium(III) nitrate hexahydrate, Ce(NO3)3 ∙ 6H2O:
6.5133g - Copper nitrate hexahydrate, Cu(NO3)2 ∙ 2.5H2O:
n ∙ 0.046525gfor n% Cu-CeO₂. - HMT, hexamethylenetetramine, (CH2)6N4:
28.0372g - Deionized water (18 MΩ)
Synthesis
- Dissolve the cerium nitrate and HMT in separate beakers, each containing 400mL deionized water.
- Add the copper nitrate to the cerium nitrate solution
- Stir both beakers for 30 minutes
- Combine the contents of each beaker in and stir for 30 minutes
- Move the mixture to a 40°C water bath and stir for 3 hours
- Remove the mixture from the water bath and stir for 18 hours

Centrifugation and Drying
The equipment used is a Beckman Coulter Avanti J-E centrifuge at Columbia Biomolecular Characterization Facility with JA-17 rotor. The samples were centrifuged at 12500 rmp for 2 hours at 4°C. After centrifugation, excess supernatant was removed and the bottles were placed in a drying bin with aluminum foil cover for 72 hours.  The dried samples were then ground into fine powders using mortar and pestle.
The dried samples were then ground into fine powders using mortar and pestle.
Pellet Pressing
The powders were pressed into pellets using a Enerpac hydraulic press at Lamont–Doherty Earth Observatory under the guidance of Professor David Walker and Supervisor Echo Shuo. 
- Place the silver housing firmly onto the black housing, and insert the first small cylinder into the chamber
- Deposit 0.170g of powder into the chamber.
- Insert the second small cylinder and the long cylinder into the chamber
- Bring die to the press, turn the oil valve to the closed position.
- Lower the hydraulic arm until it is touching the sample and incrementally increase the pressure to 5000psi
- Wait until the pressure drops to around 2000-3000psi (roughly 20-25 minutes).
- Slowly release the pressure using the valve until the hydraulic arm lifts from the die
- Turn the die upside down and place a small acrylic shield around the black housing for pushing out the central cylinders.
- Lower the hydraulic arm carefully until the silver housing barely makes contact with the press stage
- Remove the small cylinder that is now fully exposed and carefully remove the pellet
- Disassemble and thoroughly clean all parts of the die with a Kimwipe and isopropyl alcohol

Sintering
The heating program performed is as follows:
- ramp 1:
120°C/h - level 1:
350°C - duration 1:
4h - ramp 2:
120°C/h - level 2:
0°C(This will only cool the pellet to room temperature) - duration 2:
∞

Electrode Appllication
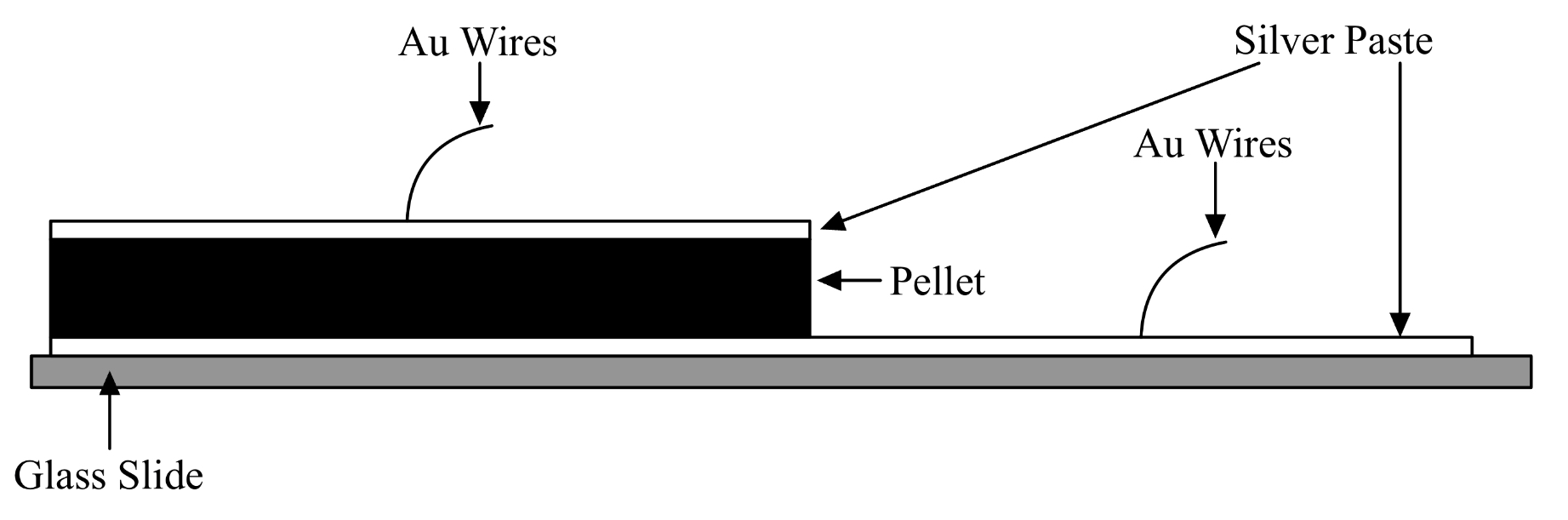
- Using a Q-tip, apply colloidal silver paste (Electron Microscopy 12640) thinly onto the top surface of each pellet
- Paint a microscope slide with a track of electrode paste for about 2cm long
- Let both dry for 2 hours
- Apply a dab of Ag paste to one end of the track, flip the pellet and stick the painted side onto the dab
- Let dry for 2 hours
- Cut gold wire into 5cm long sections. Dip one end of each wire into the Ag paste to improve cohesion with pellet/track
- Paint the other side of the pellet
- Dry for 2 hours
- Attach the painted ends of the wires to the surface of the pellet and the end of the conductive track using dabs of silver paste.
- Dry for 24 hours
Impedance Spectroscopy
Impedance spectroscopy was performed using an AMETEK SI 1200 impedance spectrometer. The furnace had an improvised faraday cage shielding to reduce noise. A cardboard box with foam inside was used to further insulate the furnace when performing measurements. 
Set Up
- Place the sample into the box furnace on top a piece of porous ceramic material
- Snake the ends of each gold wire through the holes in the contacts at the end of each copper wire
- Connect the two wires to the input ports of the impedance analyzer
- Turn on the impedance analyzer
- Turn on the furnace, set the Temp dial to 2.5 times the desired temperature
Measurement and Data
We collected EIS spectra from 1 MHz to 1 Hz with five loops per sweep, at 50 °C intervals between 50 and 200 °C, for 0–16 % Cu-doped samples (0, 1, 2, 4, 8, 16 %). Data were captured using SMaRT EIS and fitted in ZView with equivalent-circuit models; XRD and PDF analyses were used to interpret structural features. Together, these measurements indicate conductivity enhancement versus undoped ceria and behavior consistent with thermally activated small-polaron hopping. Quantitative values and model details are included in an in-review manuscript and will be added here following publication.
